Regulation and Innovation
Ian McCarthy | Emory University
Outline for today
- Innovation and R&D decisions
- MEI, dynamic efficiency, and what gets researched
- Prices, competition, and high drug spending
- U.S. vs peers, brand vs generic, limited competition
- U.S. vs peers, brand vs generic, limited competition
- Policy responses and global equity
- Price regulation, push vs pull incentives, global public good
Innovation and R&D Decisions
What is innovation?
How would you define “innovation” in pharma?
- Should not just mean a new drug, but a new drug that is better than existing treatments
- Not clear this is always the argument made in the media
Inducing Innovation
- Governments often care not just about using existing technologies efficiently, but about creating new ones
- Classic example: British Parliament’s 1714 Longitude Prize, which paid for a method to determine longitude at sea
- The prize worked: it induced decades of R&D by clockmakers, eventually yielding highly accurate marine chronometers
- Question for today: what “prizes” or incentives are we implicitly setting for pharma R&D?
What governs innovation?
- Strong empirical evidence that firms internalize future profits of a drug when making R&D decisions
- Investment decision can be modeled as:
- Ranking of expected returns to potential new drugs, including costs of manufacturing, packaging, marketing, and distribution. This is the Marginal Efficiency of Investment (MEI)
- Comparison of MEI to cost of capital
- Investment in the drugs to the point where MEI=cost of capital
MEI and the Cost of Capital

- Downward-sloping MEI: diminishing returns to additional R&D projects
- Horizontal line: cost of capital
- A leftward shift of MEI (e.g., from lower prices) reduces the profit-maximizing R&D level
Dynamic Efficiency
- Policies that lower drug prices today reduce current spending but also reduce expected future profits
- If firms internalize these future profits, lower prices → lower MEI → less R&D and fewer future drugs
- Dynamic efficiency tradeoff:
- Static gain: lower prices and better access for today’s patients
- Dynamic cost: potential reduction in future innovation
- Example: debates around Medicare drug price negotiation and the Inflation Reduction Act can be viewed through this lens
What Gets Researched? Preventives vs Treatments
- Even holding total market size fixed, incentives can differ sharply between:
- Preventive products (e.g., vaccines)
- Treatments after disease onset
- Kremer & Snyder show that with heterogeneous risk, firms may earn more from treatments than preventives, even when preventives generate more social surplus
- Intuition: high-risk patients are easier to identify and treat; low-risk individuals may not buy prevention at a price that fully reflects its social value
Kremer–Snyder Stylized Example
- Suppose:
- Some consumers have a high probability of getting the disease; others have low risk
- A preventive reduces risk; a treatment cures the disease after it appears
- With the same underlying health risk and willingness-to-pay:
- Treatment can yield higher firm revenue than prevention because only high-risk consumers are willing to pay high prices for the preventive
- Takeaway: private R&D may tilt toward treatments over preventives, even when prevention is socially preferable
Stage of Disease and Trial Length
- Firms also choose which stage of disease to target
- Shorter clinical trials mean:
- Faster time to approval
- Longer period of effective market exclusivity
- Empirically, cancer R&D is heavily concentrated in late-stage and recurrent disease, with far fewer trials on prevention or very early-stage disease
- This suggests underinvestment in long-term, prevention-oriented projects relative to social value
Evidence: Cancer Trials by Stage
- Data on cancer clinical trials show:
- Very high numbers of trials for recurrent and metastatic disease
- Much smaller numbers for in situ cancers and especially for prevention trials
- Interpretation:
- Market incentives favor drugs with shorter, cheaper trials and quicker payoffs
- Long-horizon prevention may be dynamically underprovided
Prices, Competition, and High Drug Spending
Paper Discussion 1
The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform
1. High Spending
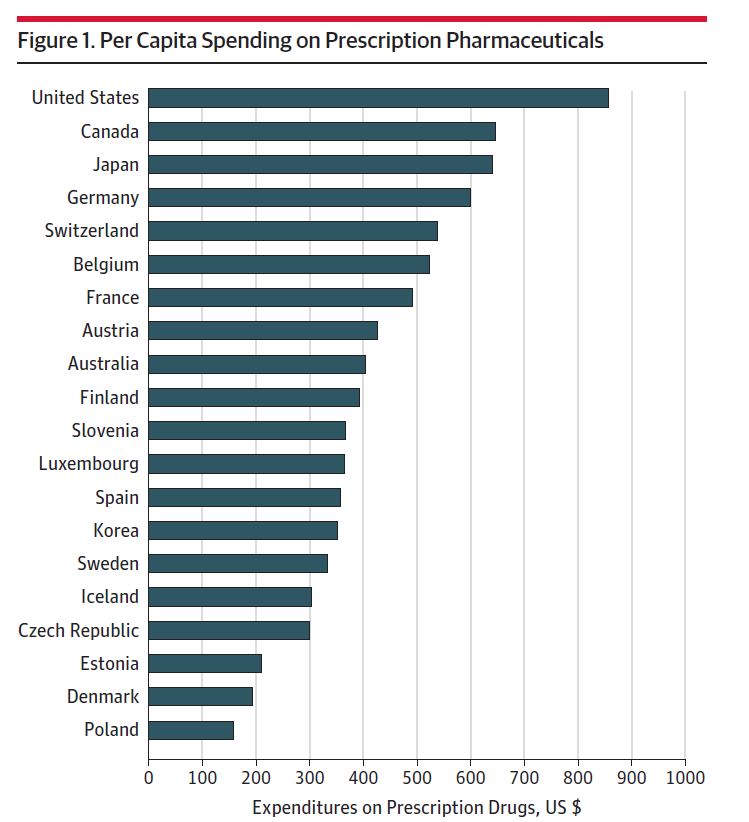
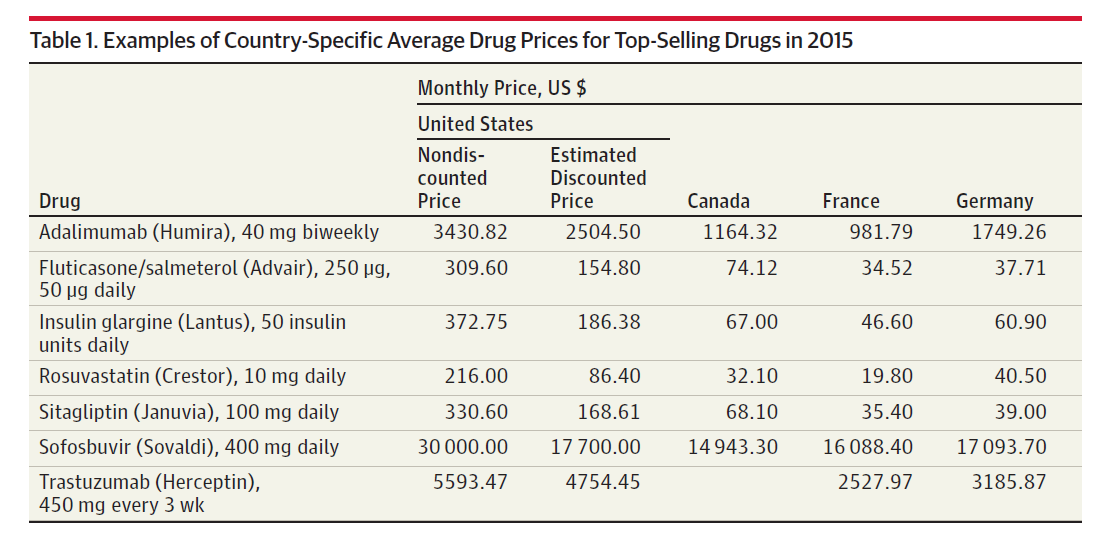
- Higher spending in the U.S. compared to peer countries
- Faster spending growth in the U.S. compared to peer countries
But…
- Spending on even very high cost drugs can still be cost-effective
- Sovaldi (hepatitis C) found to be cost-effective despite $84,000 per 12-week course
Research Questions
- What factors seem to contribute to pharma price increases?
- What policies can help ensure prices match value and that drugs are available in an equitable way?
Brand name vs generic
- Annual cost of highly specialized brand name drugs exceeds $250,000 per patient!
- High brand-name prices now extend to drugs with some substitutes (not just rare conditions)
- Generic drugs generally lower priced, but some examples of high price increases
- Daraprim, 63-year old treatment for toxoplasmosis, increases 5500% (from $13.50 to $750) in 2015
- A few hundred generic drugs experienced price increases of more than 1000% from 2008 to 2015
- Why? Monopoly products, despite no patent protection
Why such high prices?
- Prices set by manufacturers, PBMs, insurers, and pharmacies
- Little direct price regulation
- Conversely, the UK specifically requires cost-effectiveness thresholds before allowing coverage
Case Study: Avastin and Price Discrimination
- Avastin was approved for certain cancers and priced accordingly
- Physicians later discovered that very small doses were also effective for treating macular degeneration
- At the cancer price, the implied price per eye injection for ophthalmology was extremely high
- Regulatory and reimbursement rules limited Genentech’s ability to charge very different prices across indications, creating tensions around off-label use and follow-on products
- Example of how indication, dosing, and pricing regulations interact with firms’ incentives
Drug Life Cycle and Patent Clock
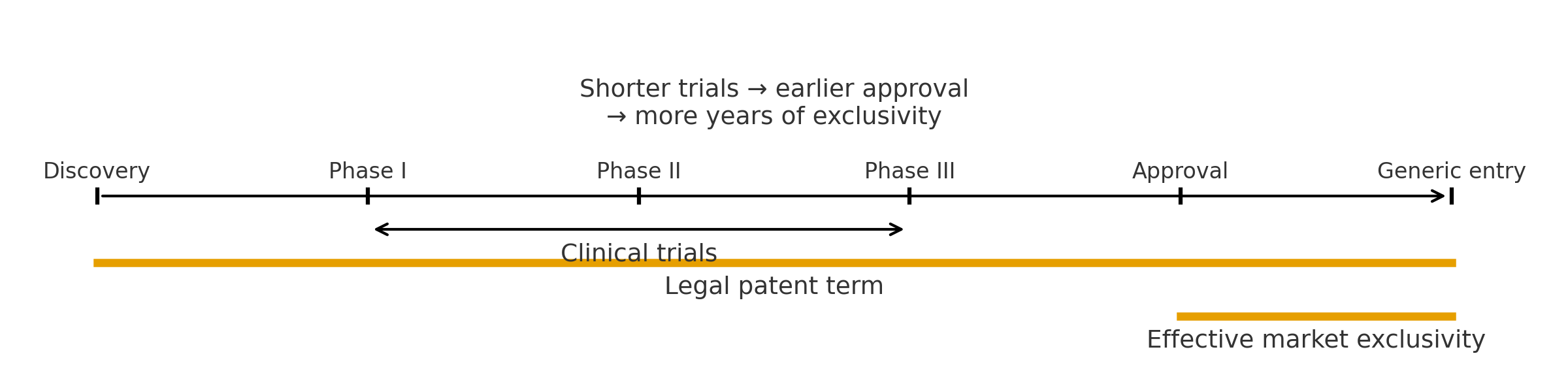
- Legal patent term starts before approval; effective market exclusivity is shorter
- Shorter clinical trials bring approval earlier and lengthen effective exclusivity
- R&D incentives are shaped by where projects sit on this timeline
Competition
- Patent protection usually lasts around 20 years (starts before drug is officially approved)
- Companies can apply for extended patent protection for 5 years (max of 14 years in the market)
- Additional 6 months if testing products in children
- On average, 12.5 years of post-market exclusivity for widely used drugs and 14.5 years for highly innovative drugs (first-in-class)
Limited competition even for brand name drugs with some substitutes. Why?
- Fragmentation
- Information problems
- Physician agency
Firms can also deter generic entry, via:
- Additional patents on coating, method of administration, etc.
- Example: Nexium is derivative of Prilosec (omeprazole), sold for 600% markup over generic omeprazole
- Significant settlements to generic drug companies
- Delays in approval for generic drugs (FDA delays of 3-4 years, now closer to 1-2 years)
Barriers to substituting brand name drugs with generic drugs even after generic entry:
- Drug product selection laws in 30 states that allow but do not require pharmacists to substitute generics
- 26 states that require patient consent when replacing brand name with generic drugs
- All states allow “dispense-as-written”, which restricts pharmacists’ ability to substitute brand name drugs
Drug Pricing Supply Chain
- Already discussed…
- Manufacturers set list prices and negotiate rebates with PBMs
- Insurers reimburse pharmacies; patients face cost sharing at the pharmacy
- PBM payment structures can weaken incentives to push aggressively for lower net prices
Other barriers to competition among generics and brand name:
- PBMs often paid based on insurer spending on specific drugs, therefore little incentive to intensely negotiate prices
- From US House Committee on Oversight and Government reform:
“The investigation revealed, for example, that Turing received ‘no pushback from payors’ when it increased ‘Chenodal price 5x… [Thiola] price 21x… [and Daraprim] price 43x.’
Justification for high prices
Industry argument that significant R&D costs necessitate higher prices
But…
- Half of the “most transformative drugs” developed (or started) in academic centers
- Many drugs also started in small companies later acquired by larger manufacturer
- “Innovation” means more than just another drug
- Current price growth simply not sustainable
Policy Responses and Global Equity
Price regulation and innovation
- Price regulation (reference pricing, direct price caps, etc.)
- In general, lower prices discourages innovation by shifting MEI inward
- How much? Some estimates of price elasticity of R&D at 0.6
- 10% price cut reduces R&D by 6%
- But what innovations are “lost”? Hard to say
How to influence innovation?
- If firms are maximizing profits, then the only way to influence innovation is to change the MEI or the cost of capital
- Shift MEI outward via:
- Changing marketing regulations (DTC advertising)
- Increasing demand for a drug (market exclusivity, coverage under public insurance)
- Shift cost of capital down via:
- Easier access to funding (tax credits, grants, etc.)
How to influence innovation?
- Which approach is “best”?
- Politically, these are NOT identical policies
- Pull incentives require no up-front costs
- Pull incentives tend to place R&D burden onto pharma customers
- Push incentives require up-front government investment
- Push incentives place burden on taxpayers
What can we do?
Federal Policies:
- Limit secondary patents for minor changes
- Combat anticompetitive practices like pay-for-delay
- Allow negotiation of prices and formulary exclusions by Medicare
- Faster time to market for generics
State Policies:
- Drug product selection laws
- Allow negotiation of prices and formulary exclusions by Medicaid
Advanced Market Commitments (AMCs)
- Governments or donors commit in advance to buy a specified quantity of a vaccine or drug at a pre-set price
- Goal: create a credible, profitable market for products that primarily benefit low-income countries
- Example: AMC for pneumococcal vaccines in low-income countries
- AMCs act like:
- A pull incentive (payment conditional on success)
- Targeted at areas where standard patent-based returns would be too low
Patent Buyouts and Delinking
- Patent buyouts:
- Government buys the patent at an auction-based price plus a markup
- Drug is then placed in the public domain (generic competition; low prices)
- Broader “delinkage” proposals:
- Replace or reduce reliance on monopoly pricing
- Reward firms through prizes or lump-sum payments tied to clinical and social value
- These ideas aim to preserve dynamic incentives for R&D while reducing static monopoly distortions in prices
Paper Discussion 2
Who Pays for Pharma R&D?
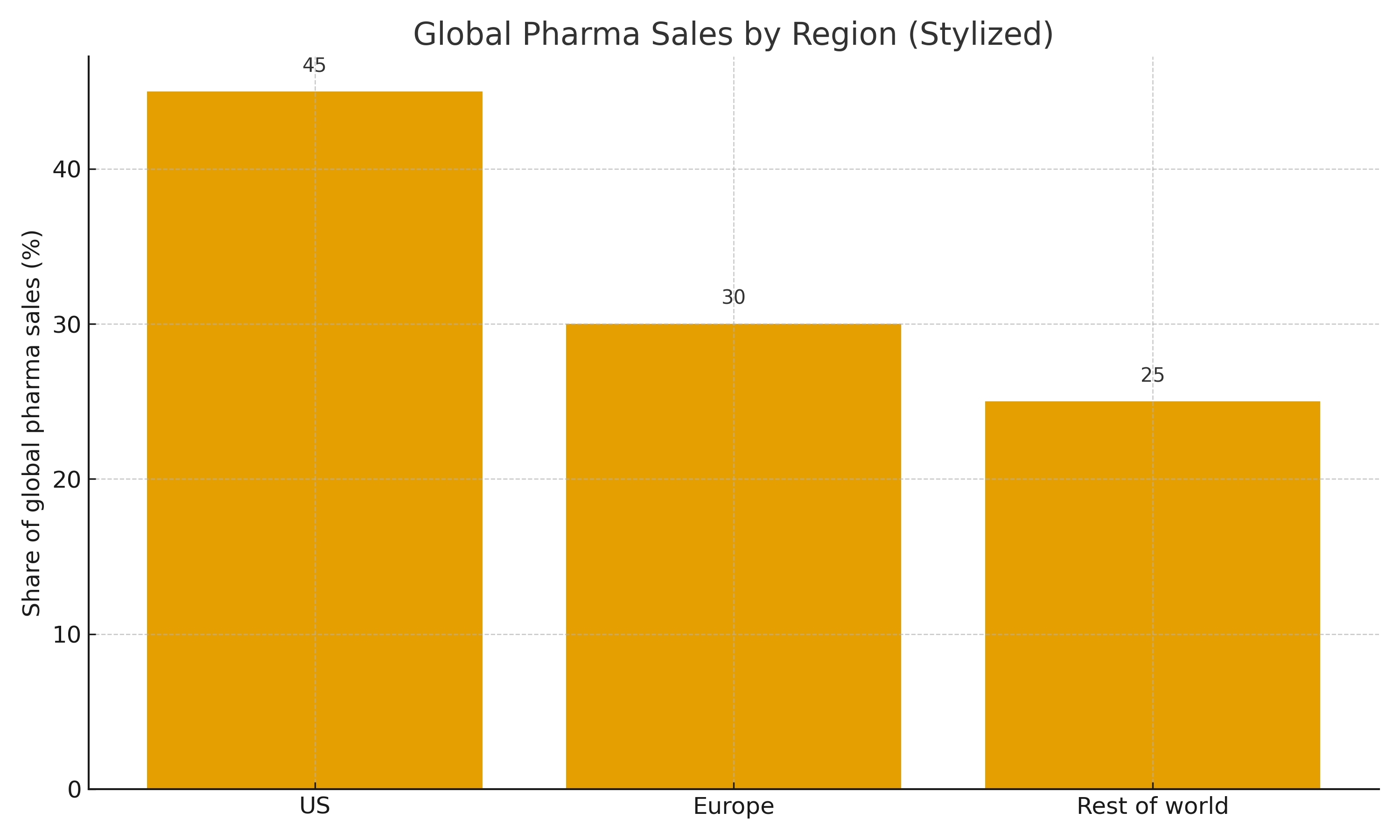
- The U.S. accounts for a disproportionate share of global pharmaceutical sales (stylized)
- Other high-income regions also contribute, but to a lesser extent
- Sets up the question of whether the U.S. is over-contributing to global R&D incentives
US Subsidizing ROW
- US clearly pays higher prices than other countries
- Strong evidence that US contribution to profit for future innovation is higher than it should be
- That said, the ROW contribution is meaningful (all other countries do not force prices to marginal costs)
- Authors urge a more international view of spending, suggesting better policy is to advocate for higher prices elsewhere rather than directly for lower prices in the US
Pulling It Together: Designing R&D Incentives
- Standard patent system: strong dynamic incentives but high prices and uneven R&D portfolio
- Distortions:
- Treatments vs preventives
- Short- vs long-horizon projects
- Diseases of rich vs poor countries
- Policy menu:
- Adjust prices and coverage (Medicare/Medicaid negotiations, global pricing)
- Add targeted push and pull incentives (NIH funding, AMCs, prizes, buyouts)
- Open question: what mix best balances current access, future innovation, and global equity?